Accelerating Biomarker Research Through Robust Immuno-based Proteomics
Reliable biomarkers are essential for early disease detection, patient stratification, and treatment monitoring. Among biomarker types, proteins offer significant advantages, enabling cost-efficient and high-throughput measurements often within minutes using standard immuno-assays or lateral flow devices. Sciomics is a leading Biomarker CRO dedicated to advancing protein biomarker research for precison medicine. We provide a comprehensive and reproducible approach to biomarker discovery and validation utilizing our scioDiscover platform. Our expertise assists researchers across diverse fields, ensuring the generation of robust and clinically relevant results.
Enabling reliable & translational Biomarker Discovery
Our scioDiscover platform ensures:
- Robust Data: Ensure data reliability and high reproducibility with four technical replicates per target protein and sample.
- Broad Coverage: Unmatched multiplexing and sensitivity for diverse, complex samples.
- Effortless translation: Seamless integration into diagnostic assays and lab tests due to immuno-based methodology..
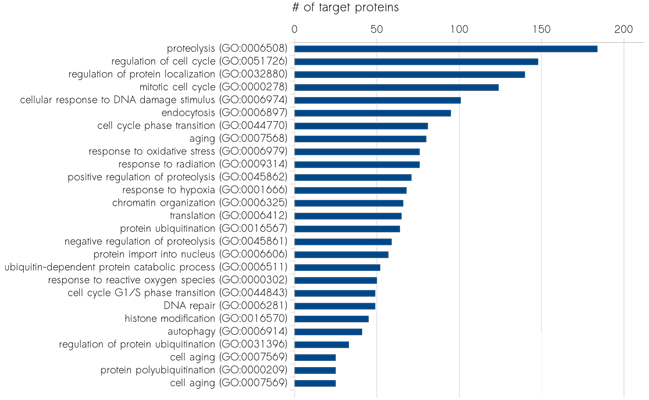
Our track record in biomarker discovery
We have performed successful biomarker studies in diverse research areas, including:
- Acute Kidney Injury (AKI)
- Assessing Dengue Fever Severity
- Neurodegenerative Diseases (Alzheimer’s & Parkinson’s)
- MCDS (Metaphyseal Chondrodysplasia, Schmid Type)
- COVID-19 disease severity
- Endometrial Cancer
- Endometriosis
- Infectious Diseases
Comprehensive Scientific Support for Protein Biomarker Discovery & Development
We offer tailored expert guidance throughout the entire process, from study design and protein analysis to data interpretation and biomarker validation. Choose Sciomics as your preferred Biomarker CRO for development projects.

1. Study design: Our experts help perfect your experimental biomarker study setup, sample, and control selection for robust, meaningful results.
2. Sample selection: We analyze diverse sample types (plasma, serum, urine, CSF, ISF, PBMC, tissue, cells, etc.), requiring minimal input amounts.
3. Sample analysis: Our experts process your samples from protein extraction to incubation, delivering results quickly (within few weeks).
4. Data analysis: Comprehensive bioinformatics and machine learning provide actionable insights, ROC-curves, and robust, interpretable biomarker panels for routine testing.
5. Data interpretation: Detailed reports and guidance to support your validation and follow-up strategies.
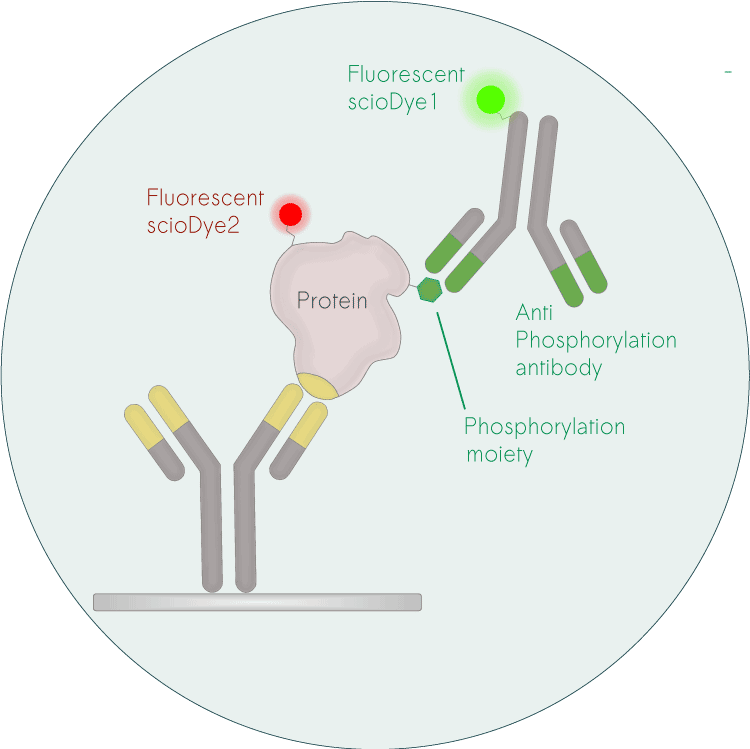
6. Validation: Our antibody platform facilitates easy transfer to standard immuno-assays, accelerating validation and diagnostic assay development.
Biomarker discovery based on our antibody-based discovery platform ensures a straight-forward translation into other immuno-assays for validation, accelerating and de-risking the development of diagnostic assays.
Discuss your project
with a scientist
Validating scioDiscover Biomarkers: Proven Success
Direct Validation: Easily confirm scioDiscover findings using common immuno-assays like ELISA.
Accelerated Implementation: Speed up the journey from biomarker discovery to clinical use and diagnostics.
Exceptional Correlation: Strong agreement (Pearson’s r > 0.9) between discovery and validation data.
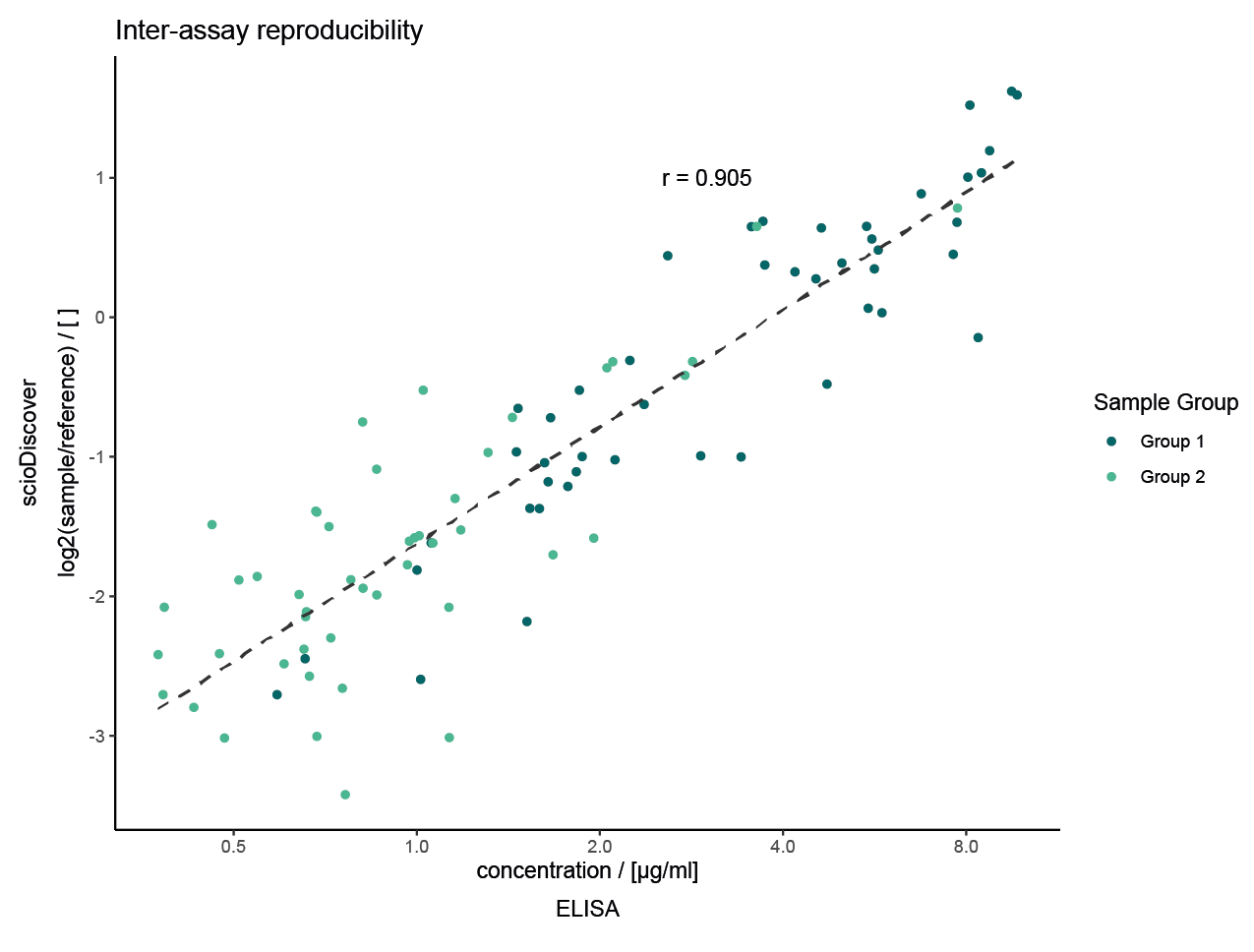
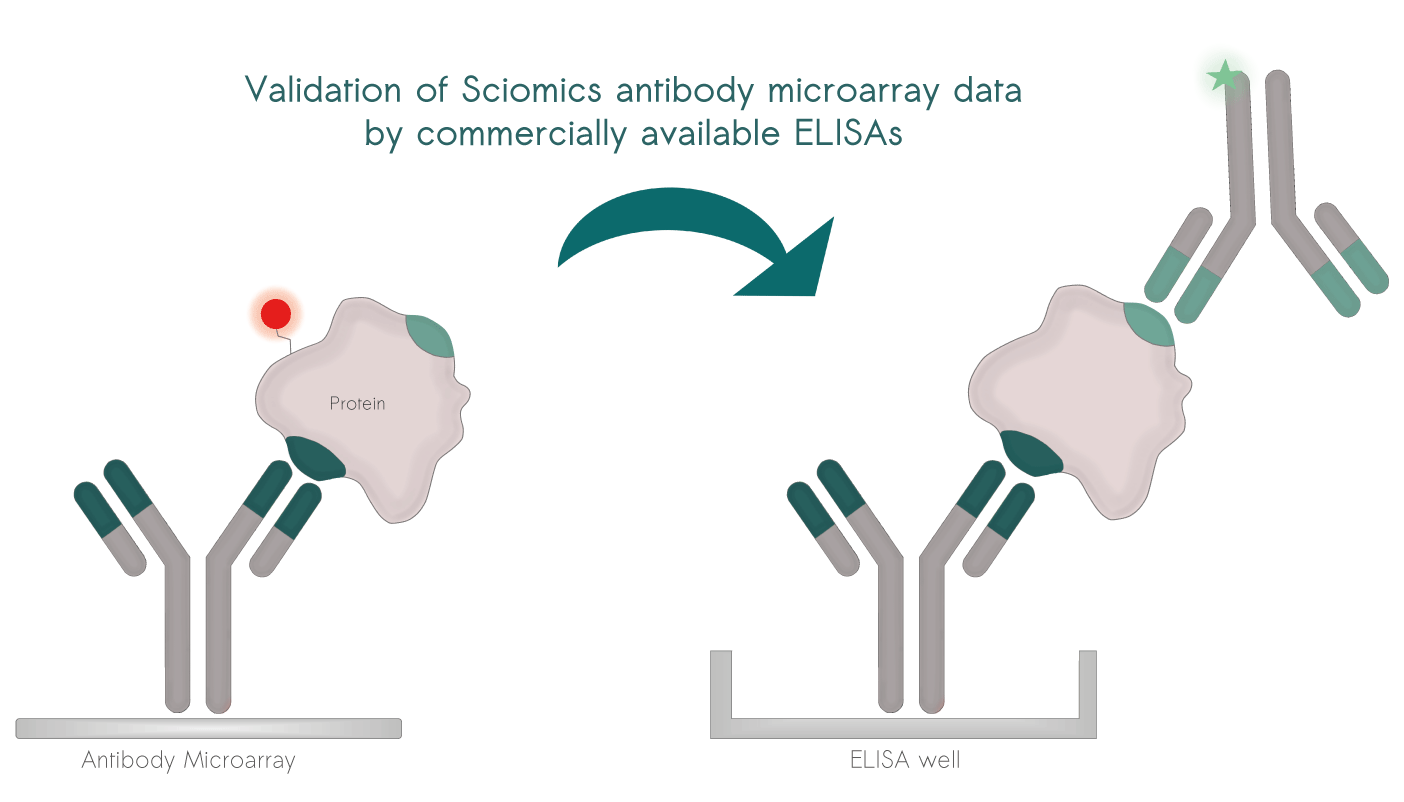
Selected projects with external validation
Roche: Validated two biomarkers using their Elecsys platform ( poster)
AbbVie: Confirmed biomarkers from a small cohort on an internal platform (paper)
University Hospital Heidelberg: Validated CRP in their clinical laboratory; additional marker confirmed by ELISA in our lab (paper)
Hannover Medical School: Validation of tissue proteomics/phosphoproteomics in a mouse model by Western Blotting (paper)
Charité: Verified phosphorylation state via Western blot/IHC in a tissue study (paper)