In recent decades, our understanding of PTMs has grown immensely, identifying over 500 distinct types to date. The most studied PTMs, representing nearly 90 % of reported PTM sites, include phosphorylation, acetylation, ubiquitination, succinylation, and methylation. Excitingly, new discoveries revealed additional modifications such as crotonylation, monoaminylation, and lactylation.
PTMs play a critical role in cellular responsiveness and adaptation. Their predominantly reversible nature allows a precise spatial and temporal control over protein chemistry and function, enabling cells to quickly adapt to environmental changes. For instance, phosphorylation, one of the best studied PTMs, activates enzymes like protein kinases, triggering distinct signaling pathways. Further, PTMs regulate gene expression, protein homeostasis, protein functions, protein-protein recognition, interactions, as well as their spatial localization. With this, PTMs significantly enhance the structural and functional diversity of proteins, impacting nearly every aspect of cellular functions. However, the complete impact of many identified PTMs remains to be fully elucidated.
PTMs play a critical role in cellular responsiveness and adaptation. Their predominantly reversible nature allows a precise spatial and temporal control over protein chemistry and function, enabling cells to quickly adapt to environmental changes.
Dysregulation of PTMs is linked to a wide spectrum of diseases, from cancer and cardiovascular conditions to neurological disorders, neurodegenerative diseases, and infectious diseases. Recent research shows that diseases are primarily associated with phosphorylation (81%), methylation (9%), and ubiquitination (4%) among PTM types. However, it is important to note that these distributions may reflect the current research focus rather than the definitive role of PTM types in disease pathology. These associations are frequently observed in neurological disorders, breast diseases, and blood-related conditions. Exploring PTMs in the context of disease remains a dynamic area of investigation, shedding light on their nuanced roles in various health conditions.
Comprehensive proteome-wide analysis methods enable systematic exploration of PTMs in health and disease. Understanding the role of PTMs in disease onset and progression is complex and requires a diverse toolset of techniques. Nowadays, most techniques require pre-processing of the specimen, such as immunoaffinity enrichment or protease digestion. Mass spectrometry (MS) is the most widely used approach for PTM site discovery, offering comprehensive qualitative and quantitative analyses. However, reliance on protein digestion to generate analyzable peptides presents certain limitations and challenges.
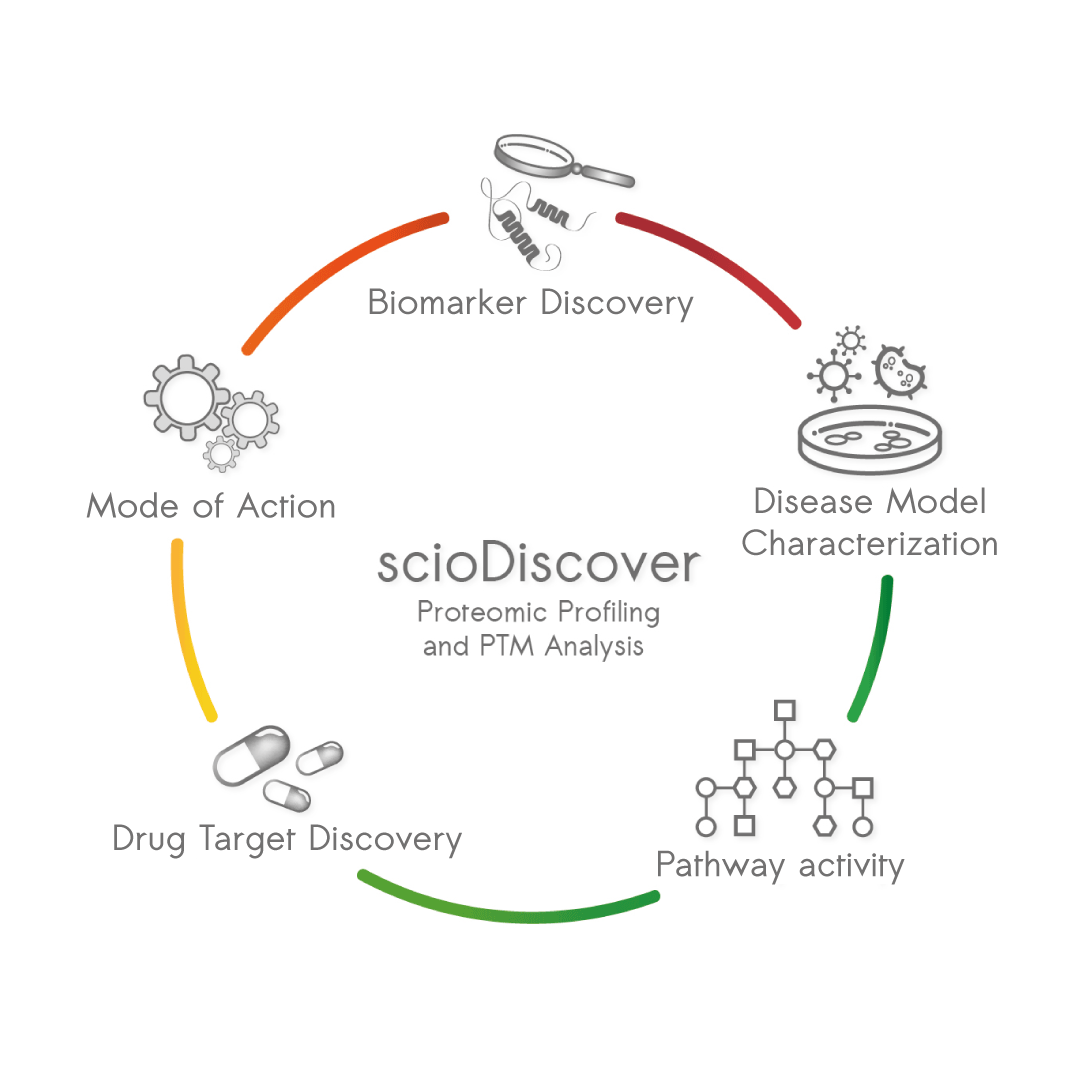
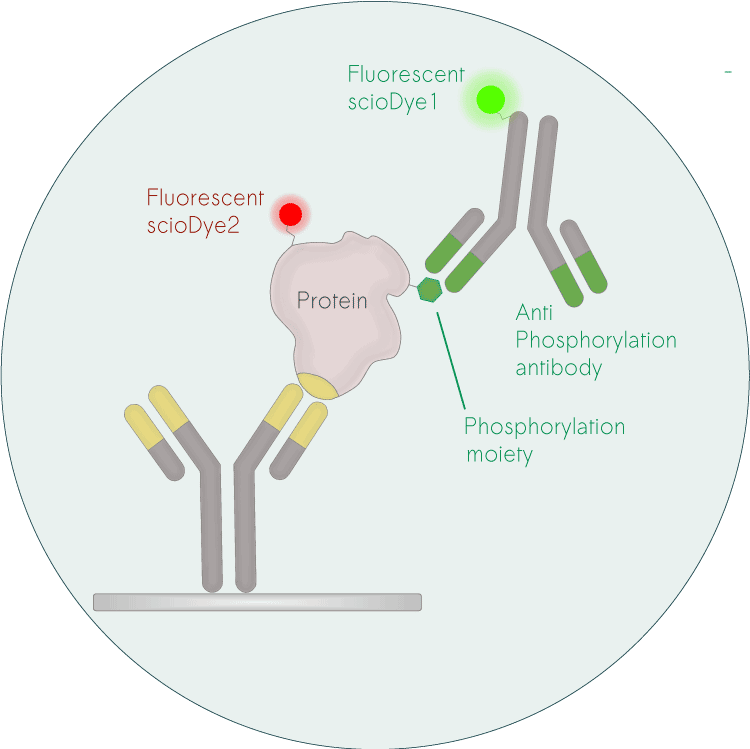
A less well-known but very successful approach is the use of antibody microarrays for a combined protein and PTM analysis. scioDiscover, a microarray-based platform by Sciomics, enables the simultaneous analysis of more than 1400 proteins, providing comprehensive insights into protein changes – without prior protein digestion, fractionation or protein-depletion and using only small sample amounts. This protein abundance profiling can be combined with a simultaneous profiling of phosphorylation status, covering serine, tyrosine, and threonine phosphorylation of all profiled proteins. The combined protein and phosphorylation-status analysis platform scioPhospho yields robust data and allows direct comparison of protein abundance and PTM-status.
Phospho-proteome analysis has already proven its scientific value in multiple studies. Recently, scioPhospho was applied in finding a solution for cyclosporine-A (CsA)-induced anemia and kidney toxicity. CsA is an immunosuppressant and standard of care in organ transplantation, acting through inhibition of the phosphatase calcineurin. Even though CsA’s benefits are substantial, CsA may exert short- and long-term adverse effects on the kidney. Long-term CsA treatment is known to cause renal vascular constriction, occlusive afferent arteriolopathy, nephron ischemia, and striped tubular-interstitial fibrosis. Therefore, loss of kidney function may outweigh the benefits of CsA. The pathophysiology of CsA-induced kidney injury is not completely understood, but hypoxia of nephron segments likely is involved. In their study ‘Daprodustat prevents cyclosporine-A-mediated anemia and peritubular capillary loss’, Labes et al. investigated the effect of CsA on the phospho-proteome in the kidney and identified a promising combinatorial approach for alleviating both CsA-induced anemia and kidney toxicity.
Daprodustat, a selective inhibitor of the prolyl-hydroxylase domain, has emerged as an erythropoiesis-stimulating agent, based on its ability to upregulate hypoxia-inducible factor (HIF), and thus, erythropoietin (EPO). In the study, mice were treated with CsA, Daprodustat, or in combination for up to eight weeks. CsA-induced kidney injury was assessed in the mouse model by plasma creatinine, routine histology, and kidney injury markers. Daprodustat or Daprodustat/CsA had no impact on plasma creatinine. However, following CsA treatment, focal tubular atrophy and interstitial fibrosis were observed. The histological findings, indicating CsA-induced kidney injury, were supported by multiple molecular markers, including KIM-1, NGAL, dickkopf-3 and vimentin.
Since CsA acts as phosphatase inhibitor, alterations in protein phosphorylation levels are supposed to be involved in CsA-induced kidney injury. For in-depth analysis of the phospho-proteome, the scioPhospho platform was used to analyze proteins involved in tissue injury and repair, including T-cell activation, cell cycle regulation, oxidative stress response, cell adhesion, apoptosis, and angiogenesis. CsA treatment changed the expression of 79 and the phosphorylation of 86 proteins. Interestingly, CsA downregulated both expression and phosphorylation of mTOR. Daprodustat alone had a limited effect, with altered phosphorylation levels of only two proteins. In combination, Daprodustat abolished the effect of CsA on protein phosphorylation, as only six of previously 86 proteins were differentially phosphorylated. Daprodustat had no significant effect on CsA-induced phospho-mTOR depression, and therefore, it appears unlikely that Daprodustat restored angiogenesis through mTOR. However, Daprodustat reversed the effect of CsA on phospho-PRKAR2A, another key regulator of angiogenesis. In conclusion, microarray-based phospho-proteomics revealed phospho-PRKAR2A as a potential prognostic tissue marker in CsA toxicity. Daprodustat abolished the changes in phosphorylation induced by CsA and this suppression of broad phosphorylation changes was involved in the protective action of Daprodustat after CsA treatment. Thus, by leveraging scioPhospho, the potential of Daprodustat as a promissing intervention to counteract CsA-induced kidney injury could be revealed.
Beyond phosphorylation, the antibody microarray technique provides a flexible platform which can be adapted to analyze a range of PTM types. With the scioUbi platform, Sciomics offers protein profiling alongside ubiquitination analysis. In recent years, Sciomics has dedicated significant efforts to developing and optimizing platforms for combined protein and PTM discovery, including acetylation and methylation analysis, with a focus on asymmetric and symmetric dimethylation of arginine. This fast and cost-effective analysis of various PTMs, coupled with protein level data, facilitates the profiling and identification of new PTM signatures in health and disease. Such insights into underlying biological mechanisms might not only identify potential PTM protein biomarkers but also reveal promising therapeutic targets.
Labes, R., Brinkmann, L., Kulow, V. A., Roegner, K., Mathia, S., Balcerek, B., Persson, P. B., Rosenberger, C., & Fähling, M. (2022). Daprodustat prevents cyclosporine-A-mediated anemia and peritubular capillary loss. Kidney international, 102(4), 750–765. https://doi.org/10.1016/j.kint.2022.04.025
Published: 19 June 2024