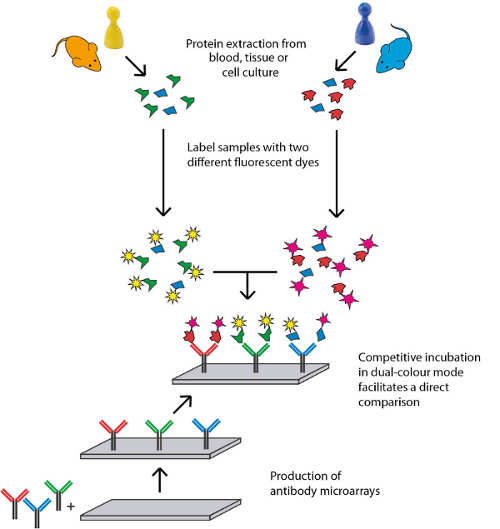
High quality standards are essential for robust and reliable biomarker discovery and validation as well as drug target discovery. Over the past 10 years, a stringent quality assurance workflow has been established for our antibody array platform. This allows us to conduct immuno-based biomedical discovery studies with excellent standards in terms of reproducibility and platform stability. The automated assay workflow further improves the performance of our service.
Biomarker Discovery Phase - scioDiscover Platform
- Stringent antigen design ensures maximum specificity of the resulting antibodies
- Antibodies are antigen affinity-purified
- Targets are identified through transcriptional studies and experts
- Stringent quality conrol (QC) management
- Features:
- Cross-species activity (human and mouse for high versatility)
- Detection of numerpous protein isoforms
- Cost-efficiency due to high content analysis
Verification Phase - Custom Array Platform
- Stringent antigen design to maximise specificity of the resulting antibodies
- Antibodies are antigen affinity-purified
- In-house QC on Western Blot (different cell lines or tissues) and optional ELISA-based tests
- In-house QC on large-scale protein arrays (> 9.000 human proteins)
- In-depth validation of array performance
- Definition of biomarker signature with highest accuracy
- Selection of appropriate binders / sandwich pairs
- Features:
- Specifically designed antibodies for further use in diagnostic assays
- Fully renewable antibody formats
- Thoroughly quality controlled (Western blot analysis, ELISA)
- Comprehensive cross-reactivity profile (large-scale protein arrays)
Orthogonal Biomarker Validation
Verified candidates can be further validated by orthogonal methods according to customer’s requirements
- Western blot analysis (WB)
- ELISA
- Immunoprecipitation (IP)
- Mass spectrometry (MS)
- Biacore
Overview
Scio-Discover Biomarker
Discovery Custom Microarrays
for Biomarker Verification Contact
us