Powering breakthrough research using Multiomics

Powering breakthrough research using Multiomics
Singleron and Sciomics – Collaborating in the pursuit of Precision Medicine
Singleron Biotechnologies and Sciomics have a common goal: Precision Medicine. Their scientific approaches, however, seem to come from two sides of a coin. Consequently, their strategic alliance is expected to reach complementary and interlocking scientific findings in the quest of holistic disease research.
Cologne and Heidelberg, Germany, 30 May 2023
Singleron Biotechnologies, a front-runner in single cell multi-omics sequencing solutions and Sciomics, a first-class provider of explorative proteomic and post translational modification profiling platforms, announce their collaboration to advance Precision Medicine.
Singleron Biotechnologies is an innovative single cell sequencing solution provider with a focus on molecular diagnostics. They apply ground-breaking single cell analysis techniques for clinical diagnosis, drug development, and health management. Sciomics’ powerful proteomic and post-translational modification profiling platform provides in-depth insights on protein levels to clients from biotech and pharma industry and is internally used to develop innovative biomarker signatures.
“At Singleron we believe that single cell sequencing and analysis is vital to understand complex disease mechanisms to evolve increasingly individualized medicine” said Dr. Andreas Schmidt, Senior Vice President of Global Business Development at Singleron. “This new relationship between Singleron and Sciomics offers a synergistic approach to understand the multiple layers of cellular regulation”.
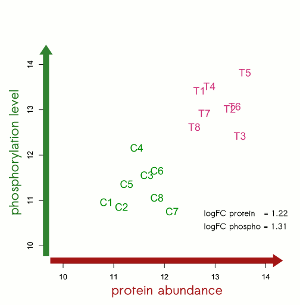
“Over the last decade we developed an efficient and robust platform for profiling protein levels and phosphorylation status.”, said Dr. Christoph Schröder, Chief Executive Officer at Sciomics. “By combining multiple omics technologies, we believe significant advances can be facilitated in the area of precision medicine.”
Together, their strategically combined expertise integrates single cell analyses with cutting-edge technology for biomarkers, profiling pathway activity and soluble markers and is expected to deliver innovative and novel insights accelerating advancements in personalized medicine promoting human health.
About Singleron
Founded in 2018, Singleron develops and commercializes single cell multi-omics products that can be used in both research and clinical settings. Its current product portfolio includes instruments, microfluidic devices, reagents, software analysis and database solutions that facilitate high-throughput single cell analysis. Singleron’s sample services offer expert execution and generation of high-quality results for academia, clinics, and biotech.
The company currently has offices, laboratories, and manufacturing facilities in Germany, Singapore, China, and the US. Its products are used in over 2,000 laboratories in hospitals, research institutes, and pharmaceutical companies.
About Sciomics
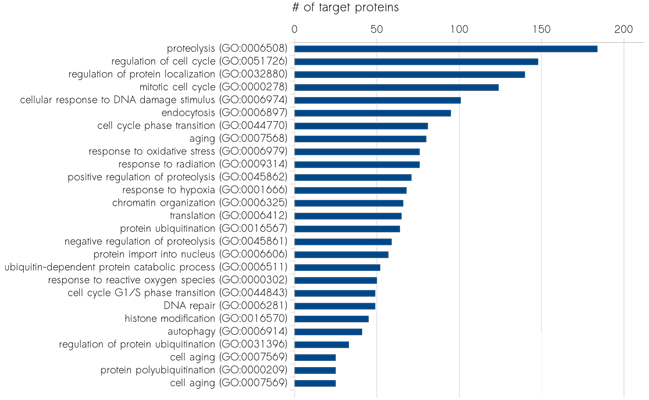
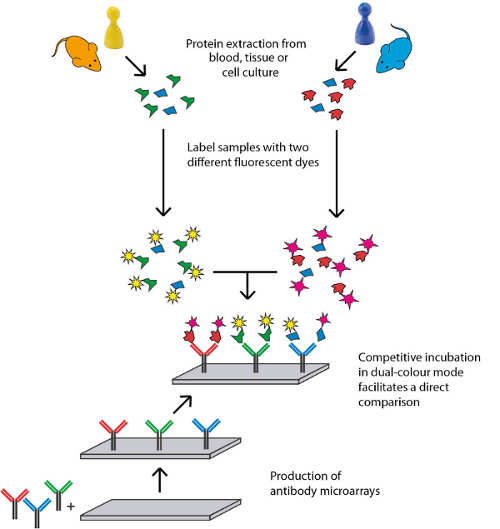
Sciomics has extensive expertise in the field of biomarker discovery, in vitro and in vivo. Their model system characterisation and disease mechanism profiling use high-content protein and post-translational modification profiling. Currently, this results in over 1,400 analysed proteins by using their proprietary fully immuno-based scioDiscover antibody array platform in a single assay with minimal sample requirements combined with Machine Learning assisted data analysis. The high-content and high-throughput platform guarantees robust and reproducible results which can be easily translated into validation and clinical assays. Sciomics serves customers world-wide, focussing on the European and North American market.
Media contacts
This email address is being protected from spambots. You need JavaScript enabled to view it.
This email address is being protected from spambots. You need JavaScript enabled to view it.