Heidelberg, 12th February, 2018
Multinational consortium has been granted 5.7 Million Euro for the development of novel therapeutic approaches in rare skeletal disorders. Sciomics' expertise with the antibody microarray technology herein serves as a valuable tool for biomarker identification and personalized treatment monitoring.
The biotechnology company Sciomics GmbH, located in Heidelberg, Germany, today announced its participation in the multinational clinical project 'MCDS Therapy'. This five-year collaborative study comprises both world-renowned clinical centres as well as small and medium-sized enterprises in the EU and Australia with a total funding of 5.7 Million Euro. In successive clinical trials, the re-purposing of the drug carbamazepine (CBZ) for the treatment of the skeletal disease metaphyseal chondrodysplasia type Schmid (MCDS) will be investigated. Furthermore, 'MCDS Therapy' encompasses biomarker development and health economics assessment studies to deliver an innovative and affordable therapy for MCDS, along with diagnostic and prognostic tools to personalize the treatment.
MCDS is a skeletal disorder resulting in short stature, abnormally shortened or bowed limbs, chronic pain and decreased mobility. Less than 1 in 100,000 people is affected, which renders the disease a so-called orphan disease. The disease is incurable, and MCDS patients rely on the long-term use of pain therapy and usually undergo numerous orthopaedic surgical interventions to correct knee and hip deformities. This burden in pain and disability leads to poor quality of life and extensive healthcare costs.
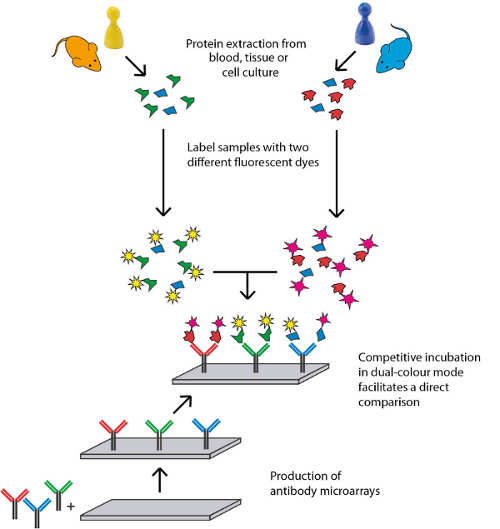
CBZ received orphan drug designation by the European Commission for the treatment of MCDS in 2016. 'MCDS Therapy' aims at advancing the re-purposing of CBZ to deliver the first non-surgical therapeutic intervention for MCDS. During the course of the project, an assessment of relevant biomarkers will be incorporated. The aim is to discover novel biomarkers that are directly relevant to CBZ treatment of MCDS which can then be used to monitor therapy and are potentially applicable to a broader group of genetic skeletal diseases or human connective tissue disease in general. 'MCDS Therapy' will use a high-content screening approaches to discover panels of potential biomarkers using samples from both mouse models and patient-derived samples. Herein, Sciomics' antibody microarrays serve as a crucial tool to identify and validate biomarkers for diagnosis, prognosis and personalized therapy.
Sciomics GmbH was founded in 2013 as a spin-off company of the German Cancer Research Centre (DKFZ). 'We are glad to participate in this highly innovative project with our solid expertise in antibody microarrays', states Dr. Christoph Schroeder, CEO of Sciomics. 'Accounting for the hallmarks of our microarray technology, which include high throughput, flexibility, multiplex analysis and robustness, I am convinced that our contribution to this novel treatment option for MCDS will be of great service. Especially for the patients suffering from this severe illness.'
> Download press release as PDF-file
Sciomics an klinischen Studien für die Behandlung von Metaphysärer Chondrodysplasie Typ Schmid (MCDS) beteiligt
Multinationales Konsortium mit einem Gesamtbudget von 5,7 Millionen Euro entwickelt neue therapeutische Ansätze für seltene Knochenerkrankungen. Hierbei spielen die Antikörper-Microarrays von Sciomics eine zentrale Rolle bei der Identifizierung von Biomarkern und Personalisierung der Therapie.
Das Biotechnologie-Unternehmen Sciomics GmbH mit Sitz in Heidelberg teilte heute seine Beteiligung an dem multinationalen klinischen Projekt „MCDS Therapy” mit. An dem Kooperationprojekt mit einem Gesamtbudget von 5,7 Millionen Euro über fünf Jahre arbeiten sowohl renommierte klinische Zentren als auch kleinere und mittelständische Unternehmen aus der EU und Australien mit. In mehreren klinischen Studien soll hierbei die Verwendung des Medikamentes Carbamazepin (CBZ) zur Behandlung der Knochenerkrankung Metaphysäre Chondrodysplasie Typ Schmid (MCDS) untersucht werden. Ein wichtiger Teil der Studien ist die Identifizierung und Validierung von Biomarkern für Diagnose, Prognose und personalisierte Therapie sowie gesundheitswirtschaftliche Aspekte, um eine innovative und kostengünstige Therapie für MCDS zu entwickeln.
MCDS ist eine Knochenerkrankung, deren Symptome Kleinwüchsigkeit, verkürzte und gekrümmte Extremitäten, chronische Schmerzen und eine eingeschränkte Beweglichkeit sind. Weniger als eine von 100.000 Personen ist davon betroffen; es handelt sich bei MCDS somit um eine sogenannte „Orphan Disease“. Die Krankheit ist unheilbar; MCDS-Patienten sind somit ihr Leben lang auf Schmerztherapie sowie orthopädische Behandlung angewiesen. Die chronischen Schmerzen und Behinderungen führen bei den Patienten zu einer geringen Lebensqualität und hohen Behandlungskosten.
CBZ hat 2016 die „Orphan Drug Designation“ der Europäischen Kommission für MCDS erhalten. Ziel von „MCDS Therapy“ ist es, mit der Verwendung von CBZ die erste nicht-invasive Therapie für MCDS zu entwickeln.Ein wichtiger Aspekt des Projekts ist die Identifizierung und Validierung von Biomarkern, die eine direkte Relevanz für die CBZ-Behandlung von MCDS haben. Diese könnten für die Therapieüberwachung von MCDS eingesetzt werden und möglicherweise auch auf andere genetisch bedingte Knochen- und Bindegewebserkrankungen übertragen werden. Im Zuge von „MCDS Therapy“ werden sowohl Proben aus Mausmodellen, als auch von MCDS-Patienten untersucht. Hierbei dienen die Antikörper-Microarrays von Sciomics als wichtige Werkzeuge bei der Identifizierung und Validierung von Biomarkern für Diagnose, Prognose und personalisierte Therapie.
Sciomics wurde 2013 als Spin-Off des Deutschen Krebsforschungszentrums (DKFZ) gegründet. „Wir freuen uns sehr, mit unserer langjährigen Microarray-Expertise zu diesem hochinnovativen Projekt beitragen zu können“, sagt Dr. Christoph Schröder, Geschäftsführer der Sciomics GmbH. „Angesichts der Vorteile unserer Antikörper-Microarrays, wie hoher Durchsatz, Flexibilität, hochparallele Analyse und Reproduzierbarkeit, werden wir einen wichtigen Beitrag zu neuen Therapieoptionen für MCDS leisten.”

Sciomics GmbH
Dr. Christoph Schröder
Im Neuenheimer Feld 583
D-69120 Heidelberg
Germany
Phone: +49 (0) 6221 42948-30
Fax : +49 (0) 6221 42948-34
Email: This email address is being protected from spambots. You need JavaScript enabled to view it.
Web: http://www.sciomics.de